Electrochemistry Multiple Choice Questions and Answers
Question 1. The following limiting molar conductivities are given:
- \(\lambda_{m\left(\mathrm{H}_2 \mathrm{SO}_4\right)}^{\circ}=x \mathrm{Scm}^2 \mathrm{~mol}^{-1}\)
- \(\lambda_{m\left(\mathrm{~K}_2 \mathrm{SO}_4\right)}^{\circ}=y \mathrm{~S} \mathrm{~cm}^2 \mathrm{~mol}^{-1}\)
- \(\lambda_{m\left(\mathrm{CH}_3 \mathrm{COOK}\right)}^{\circ}=z \mathrm{Scm}^2 \mathrm{~mol}^{-1}\)
- \(\lambda_m^{\circ}\) (in S \(\mathrm{cm}^2 \mathrm{~mol}^{-1}\)) for \(\mathrm{CH}_3 \mathrm{COOH}\) will be
- \(x-y+2 z\)
- \(x+y-z\)
- \(x-y+z\)
- \(\frac{(x-y)}{2}+z\)
Answer: 4. \(\frac{(x-y)}{2}+z\)
According to Kohlrausch’s law, \(\lambda_m^{\circ}\) for \(\mathrm{CH}_3 \mathrm{COOH}=\lambda_{\mathrm{CH}_3 \mathrm{COO}^{-}}^{\circ}+\lambda_{\mathrm{H}^{+}}^{\circ}\)
⇒ \(\lambda^{\circ}\) for \(\mathrm{H}_2 \mathrm{SO}_4=2 \lambda_{\mathrm{H}^{+}}^{\circ}+\lambda_{\mathrm{SO}_4^{2-}}^{\circ}=x \mathrm{~S} \mathrm{~cm}^2 \mathrm{~mol}^{-1}\)……(1)
⇒ \(\lambda^{\circ}\) for \(\mathrm{K}_2 \mathrm{SO}_4=2 \lambda_{\mathrm{K}^{+}}^{\circ}+\lambda_{\mathrm{SO}_4^{2-}}^{\circ}=y \mathrm{Scm}^2 \mathrm{~mol}^{-1}\)…..(2)
⇒ \(\lambda^{\circ}\) for \(\mathrm{CH}_3 \mathrm{COOK}=\lambda_{\mathrm{CH}_3 \mathrm{COO}^{-}}^{\circ}+\lambda_{\mathrm{K}^{+}}^{\circ}=z \mathrm{Scm}^2 \mathrm{~mol}^{-1}\)…(3)
On adding equation (1) and 2 x (3) and subtracting (2), we get
⇒ \(2 \lambda_{\mathrm{H}^{+}}^{\circ}+\lambda_{\mathrm{SO}_4^{2-}}^{\circ}+2 \lambda_{\mathrm{CH}_3 \mathrm{COO}^{-}}^{\circ}+2 \lambda_{\mathrm{K}^{+}}^{\circ}-2 \lambda_{\mathrm{K}^{+}}^{\circ}-\lambda_{\mathrm{SO}_4^{2-}}^{\circ}=x+2 z-y\)
⇒ \(2 \lambda_{\mathrm{H}^{+}}^{\circ}+2 \lambda_{\mathrm{CH}_3 \mathrm{COO}^{-}}^{\circ}=x+2 z-y\)
⇒ \(\lambda_{\mathrm{H}^{+}}^{\circ}+\lambda_{\mathrm{CH}_3 \mathrm{COO}^{-}}^{\circ}=\frac{(x-y)}{2}+z\)
Read And Learn More Class 12 Chemistry MCQs
Question 2. The molar conductivity of a 0.5 mol/dm³ solution of AgNO3 with electrolytic conductivity of 5.76 x 10-3 S cm-1 at 298 K is
- 2.88 S cm²/mol
- 11.52 S cm²/mol
- 0.086 S cm²/mol
- 28.8 S cm²/mol
Answer: 2. 11.52 S cm²/mol
⇒ \(\Lambda_m=\frac{\kappa \times 1000}{\mathrm{Molarity}(M)}\)
= \(\frac{5.76 \times 10^{-3} \mathrm{Scm}^{-1} \times 1000}{0.5 \mathrm{~mol} \mathrm{~cm}^{-3}}=11.52 \mathrm{~S} \mathrm{~cm}^2 \mathrm{~mol}^{-1}\)
PSEB Class 12 Chemistry Electrochemistry MCQs
Question 3. At 25 °C molar conductance of 0.1 molar aqueous solution of ammonium hydroxide is 9.54 ohm-1 cm² mol-1 and at infinite dilution its molar conductance is 238 ohm-1 cm² mol-1. The degree of ionization of ammonium hydroxide at the same concentration and temperature is
- 4.008%
- 40.800%
- 2.080%
- 20.800%
Answer: 1. 4.008%
Degree of dissociation \((\alpha)=\frac{\text { Molar conductivity at conc. } C\left(\Lambda_m^c\right)}{\text { Molar conductivity at infinite dilution }\left(\Lambda_m^{\infty}\right)}\)
α= \(\frac{9.54 \Omega^{-1} \mathrm{~cm}^2 \mathrm{~mol}^{-1}}{238 \Omega^{-1} \mathrm{~cm}^2 \mathrm{~mol}^{-1}}=0.04008=4.008 \%\)
Question 4. Limiting molar conductivity of (i.e., \(\Lambda_{m(\mathrm{NH}_4 \mathrm{OH})}^{\circ}\)) is equal to
- \(\Lambda_{m\left(\mathrm{NH}_4 \mathrm{Cl}\right)}^{\circ}+\Lambda_{m(\mathrm{NaCl})}^{\circ}-\Lambda_{m(\mathrm{NaOH})}^{\circ}\)
- \(\Lambda_{m(\mathrm{NaOH})}^{\circ}+\Lambda_{m(\mathrm{NaCl})}^{\circ}-\Lambda_{m\left(\mathrm{NH}_4 \mathrm{Cl}\right)}^{\circ}\)
- \(\Lambda_{m\left(\mathrm{NH}_4 \mathrm{OH}\right)}^{\circ}+\Lambda_{m\left(\mathrm{NH}_4 \mathrm{Cl}\right)}^{\circ}-\Lambda_{m(\mathrm{HCl})}^{\circ}\)
- \(\Lambda_{m\left(\mathrm{NH}_4 \mathrm{Cl}\right)}^{\circ}+\Lambda_{m(\mathrm{NaOH})}^{\circ}-\Lambda_{m(\mathrm{NaCl})}^{\circ}\)
Answer: 4. \(\Lambda_{m\left(\mathrm{NH}_4 \mathrm{Cl}\right)}^{\circ}+\Lambda_{m(\mathrm{NaOH})}^{\circ}-\Lambda_{m(\mathrm{NaCl})}^{\circ}\)
Question 5. Molar conductivities (∧°m) at infinite dilution of NaCl, HCl, and CH3COONa are 126.4, 425.9, and 91.0 S cm² mol-1 respectively. (∧°m) for CH3COOH will be
- 425.5 S cm² mol-1
- 180.5 S cm² mol-1
- 290.8 S cm² mol-1
- 390.5 S cm² mol-1
Answer: 4. 390.5 S cm² mol-1
⇒ \(\Lambda_{\mathrm{NaCl}}^{\circ}=126.4 \mathrm{~S} \mathrm{~cm}^2 \mathrm{~mol}^{-1}\)
⇒ \(\Lambda_{\mathrm{HCl}}=425.9 \mathrm{~S} \mathrm{~cm}^2 \mathrm{~mol}^{-1}\)
⇒ \(\Lambda^{\circ}{ }_{\mathrm{CH}_3 \mathrm{COONa}}=91.0 \mathrm{~S} \mathrm{~cm}^2 \mathrm{~mol}^{-1}\)
⇒ \(\Lambda_{\mathrm{CH}_3 \mathrm{COOH}}^{\circ}=\Lambda_{\mathrm{CH}_3 \mathrm{COONa}}^{\circ}+\Lambda_{\mathrm{HCl}}^{\circ}-\Lambda_{\mathrm{NaCl}}^{\circ}\)
=91.0+425.9-126.4
= \(390.5 \mathrm{~S} \mathrm{~cm}^2 \mathrm{~mol}^{-1}\)
Electrochemistry Multiple Choice Questions PSEB Class 12

Question 45. An increase in equivalent conductance of a strong electrolyte with dilution is mainly due to
- Increase in ionic mobility of ions
- 100% Ionisation of electrolyte at normal dilution
- Increase in both i.e., Number of ions and ionic mobility of ions
- Increase in number of ions.
Answer: 1. Increase in ionic mobility of ions
Strong electrolytes are completely ionized at all concentrations. On increasing dilution, the no. of ions remains the same but the ionic mobility increases, and the equivalent conductance increases.
Question 6. Which of the following expressions correctly represents the equivalent conductance at infinite dilution of Al2(SO4)3? Given that \(\Lambda_{\mathrm{Al}^{3+}}^{\circ} \text { and } \Lambda_{\mathrm{SO}_4^{2-}}^{\circ}\) are the equivalent conductances at infinite dilution of the respective ions.
- \(2 \Lambda_{\mathrm{Al}^{3+}}^{\circ}+3 \Lambda_{\mathrm{SO}_4^{2-}}^{\circ}\)
- \(\Lambda_{\mathrm{Al}^{3+}}^{\circ}+\Lambda_{\mathrm{SO}_4^{2-}}^{\circ}\)
- \(\left(\Lambda_{\mathrm{Al}^{3+}}^0+\Lambda_{\mathrm{SO}_4^{2-}}^{\circ}\right) \times 6\)
- \(\frac{1}{3} \Lambda_{\mathrm{Al}^{3+}}^{\circ}+\frac{1}{2} \Lambda_{\mathrm{SO}_4^{2-}}^{\circ}\)
Answer: 2. \(\Lambda_{\mathrm{Al}^{3+}}^{\circ}+\Lambda_{\mathrm{SO}_4^{2-}}^{\circ}\)
At infinite dilution, when dissociation is complete, each ion makes a definite contribution towards the molar conductance of the electrolyte irrespective of the nature of the other ion with which it is associated.
Hence, \(\Lambda_{\mathrm{Al}_2\left(\mathrm{SO}_4\right)_3}=\Lambda_{\mathrm{Al}^{3+}}^0+\Lambda_{\mathrm{SO}_4^{2-}}^0\)
Question 7. The equivalent conductance of the M/32 solution of a weak monobasic acid is 8.0 mho cm² and at infinite dilution is 400 mho cm². The dissociation constant of this acid is
- 1.25 x 10-6
- 6.25 x 10-4
- 1.25 x 10-4
- 1.25 x 10-5
Answer: 4. 1.25 x 10-5
Given, \(\Lambda=8 \mathrm{mho}^2, \Lambda^{\infty}=400 \mathrm{mho} \mathrm{cm}^2\)
Degree of dissociation, \(\alpha=\frac{\Lambda}{\Lambda^{\infty}} \Rightarrow \alpha=\frac{8}{400}=2 \times 10^{-2}\)
Dissociation constant, \(\mathrm{K}=\mathrm{Ca}^2\)
Given, C=M/32
∴ K = \(\frac{1}{32} \times 2 \times 10^{-2} \times 2 \times 10^{-2}=1.25 \times 10^{-5}\)
Electrochemistry Multiple Choice Questions PSEB Class 12
Question 8. Kohlrauschs law states that at
- Infinite dilution, each ion makes a definite contribution to the conductance of an electrolyte whatever the nature of the other ion of the electrolyte
- In infinite dilution, each ion makes a definite contribution to the equivalent conductance of an electrolyte, whatever the nature of the other ion of the electrolyte
- In finite dilution, each ion makes a definite contribution to the equivalent conductance of an electrolyte, whatever the nature of the other ion of the electrolyte
- Infinite dilution each ion makes a definite contribution to the equivalent conductance of an electrolyte depending on the nature of the other ion of the electrolyte.
Answer: 1. Infinite dilution, each ion makes a definite contribution to the conductance of an electrolyte whatever be the nature of the other ion of the electrolyte
At infinite dilution, when dissociation is complete each ion makes a definite contribution towards the molar conductance of the electrolyte irrespective of the nature of the other ion with which it is associated and that the molar conductance of any electrolyte at infinite dilution is given by the sum of the contributions of two ions.
This is called Kohl Rausch’s law \(\Lambda_m^{\infty}=\Lambda_{+}^{\infty}+\Lambda_{-}^{\infty}\),
where, \(\Lambda_{+}^{\infty}\) and \(\Lambda_{-}^{\infty}\) are molar ionic conductance at infinite dilution for cation and anion, respectively.
Question 9. Equivalent conductances of Ba2+ and Cl– ions are 127 and 76 ohm-1 cm-1 eq-1 respectively. The equivalent conductance of BaCl2 at infinite dilution is
- 139.5
- 101.5
- 203
- 279
Answer: 1. 139.5
⇒ \(\lambda_{\infty}=\frac{1}{n_{+}} \lambda_{+}^{\infty}+\frac{1}{n_{-}} \lambda_{-}^{\infty}\)
So, \(\lambda_{\infty}\left(\mathrm{BaCl}_2\right)=\frac{1}{2} \times \lambda_{\mathrm{Ba}^{2+}}^{\infty}+\frac{1}{1} \times \lambda_{\mathrm{Cl}^{-}}^{\infty}\)
= \(\frac{1}{2} \times 127+76=63 \cdot 5+76=139 \cdot 5\)
Question 10. The specific conductance of a 0.1 N KCl solution at 23 °C is 0.012 ohm-1 cm-1. The resistance of the cell containing the solution at the same temperature was found to be 55 ohms. The cell constant will be
- 0.918 cm-1
- 0.66 cm-1
- 1.142 cm-1
- 1.12 cm-1
Answer: 2. 0.66 cm-1
k = \(0.012 \mathrm{ohm}^{-1} \mathrm{~cm}^{-1}\)
R = \(55 \mathrm{ohm} \Rightarrow C=\frac{1}{R}=\frac{1}{55} \mathrm{ohm}^{-1}\)
Cell Constant \(\left(\frac{l}{a}\right)=\frac{\text { Specific Conductance }}{\text { Conductance }}\)
= \(\frac{0.012}{1 / 55}=55 \times 0.012=0.66 \mathrm{~cm}^{-1}\)
Question 11. On heating one end of a piece of metal, the other end becomes hot because of
- Energized electrons move to the other end
- Minor perturbation in the energy of atoms
- Resistance of the metal
- Mobility of atoms in the metal.
Answer: 1. Energised electrons moving to the other end
The conductivity of heat in metals is due to the presence of free electrons, which move due to an increase in temperature.
Class 12 Chemistry Chapter Electrochemistry MCQs
Question 12. On electrolysis of dil. sulphuric acid using a platinum (Pt) electrode, the product obtained at the anode will be
- Hydrogen gas
- Oxygen gas
- H2S gas
- SO2 gas.
Answer: 2. Oxygen gas
During the electrolysis of dilute sulphuric acid, the following reaction takes place at the anode.
⇒ \(2 \mathrm{H}_2 \mathrm{O}_{(l)} \rightarrow \mathrm{O}_{2(g)}+4 \mathrm{H}_{(a q)}^{+}+4 e^{-} ; E_{\text {cell }}^{\circ}=+1.23 \mathrm{~V}\)
i.e., \(\mathrm{O}_{2(g)}\) will be liberated at anode.
Question 13. The number of Faradays (F) required to produce 20 g of calcium from molten CaCl2 (Atomic mass of Ca = 40 g mol-1) is
- 1
- 2
- 3
- 4
Answer: 1. 1
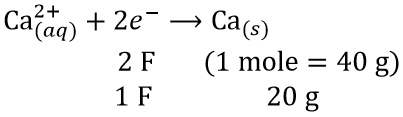
Thus, one Faraday is required to produce 20 g of calcium from molten CaCl2.
Question 14. During the electrolysis of molten sodium chloride, the time required to produce 0.10 mol of chlorine gas using a current of 3 amperes is
- 55 minutes
- 110 minutes
- 220 minutes
- 330 minutes.
Answer: 2. 110 minutes
During the electrolysis of molten sodium chloride,
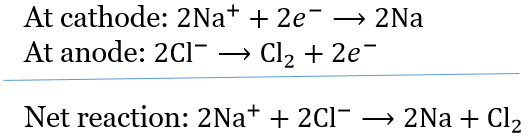
According to Faraday’s first law of electrolysis, w = Z x I x t
w = \(\frac{E}{96500} \times I \times t\)
No. of moles of \(\mathrm{Cl}_2\) gas \(\times \mathrm{Mol}\). weight of \(\mathrm{Cl}_2\) gas
= \(\frac{\text { Eq. wt. of } \mathrm{Cl}_2 \text { gas } \times I \times t}{96500}\)
0.10 x 71 = \(\frac{35.5 \times 3 \times t}{96500}\)
t = \(\frac{0.10 \times 71 \times 96500}{35.5 \times 3}=6433.33 \mathrm{sec}\)
t = \(\frac{6433.33}{60} \mathrm{~min}=107.22 \mathrm{~min} \approx 110 \mathrm{~min}\)
Class 12 Chemistry Chapter Electrochemistry MCQs
Question 15. The number of electrons delivered at the cathode during electrolysis by a current of 1 ampere in 60 seconds is (charge on electron = 1.60 x 10-19 C)
- 6 x 1023
- 6 x 1020
- 3.75 x 1020
- 7.48 x 1023
Answer: 3. 3.75 x 1020
Q = I x t
Q = 1 x 60 = 60C
Now, 1.60 x 10-19 C ≡ 1 electron
∴ 60 C \(\equiv \frac{60}{1.6 \times 10^{-19}}=3.75 \times 10^{20}\) electrons
Question 16. When 0.1 mol MnO42- is oxidized, the quantity of electricity required to completely oxidize MnO2-4 to MnO–4 is
- 96500 C
- 2 x 96500 C
- 9650 C
- 96.50 C
Answer: 3. 9650 C
The oxidation reaction is \(\stackrel{+6}{\mathrm{MnO}_4^{2-}} \longrightarrow \stackrel{+7}{\mathrm{MnO}_4^{-}}+\underset{0.1 \mathrm{~mol}}{e^{-}}\)
Q = 0.1 x F= 0.1 x 96500 C = 9650 C
Question 17. The weight of silver (at. wt. = 108) displaced by a quantity of electricity which displaces 5600 mL of O2 at STP will be
- 5.4 g
- 10.8 g
- 54.0 g
- 108.0 g
Answer: 4. 108.0 g
According to Faraday’s second law, \(\frac{W_{\mathrm{Ag}}}{E_{\mathrm{Ag}}}=\frac{W_{\mathrm{O}_2}}{E_{\mathrm{O}_2}}\) or \(\frac{W_{\mathrm{Ag}}}{108}=\frac{\frac{5600}{22400} \times 32}{8}\)
or \(\frac{W_{\mathrm{Ag}}}{108}=\frac{8}{8} \Rightarrow W_{\mathrm{Ag}}=108 \mathrm{~g}\)
Question 18. How many grams of cobalt metal will be deposited when a solution of cobalt(2) chloride is electrolyzed with a current of 10 amperes for 109 minutes? (1 Faraday = 96,500 C; Atomic mass of Co = 59 u)
- 4.0
- 20.0
- 40.0
- 0.66
Answer: 2. 20.0
w = \(\frac{I t E}{96500}\)
= \(\frac{10 \times 109 \times 60 \times 59}{96500 \times 2}=19.99=20 \mathrm{~g}\)
PSEB Class 12 Chemistry Electrochemistry Questions and Answers
Question 19. Al2O3 is reduced by electrolysis at low potentials and high currents. If 4.0 x 104 amperes of current is passed through molten Al2O3 for 6 hours, what mass of aluminum is produced? (Assume 100% current efficiency, at. mass of Al = 27 g mol-1)
- 8.1 x 104 g
- 2.4 x 105 g
- 1.3 x 104 g
- 9.0 x 103 g
Answer: 1. 8.1 x 104 g
Applying E = Z x 96500
⇒ \(\frac{27}{3}=Z \times 96500 \Rightarrow Z=\frac{9}{96500}\)
Now applying the formula, w = \(Z \times I \times t\)
w = \(\frac{9}{96500} \times 4 \times 10^4 \times 6 \times 60 \times 60=8.1 \times 10^4 g\)
Question 20. 4.5 g of aluminum (at. mass 27 amu) is deposited at the cathode from Al3+ solution by a certain quantity of electric charge. The volume of hydrogen produced at STP from H+ ions in solution by the same quantity of electric charge will be
- 44.8 L
- 22.4 L
- 11.2 L
- 5.6 L
Answer: 4. 5.6 L
We know that 1 Faraday charge liberates 1 eq. ofsubstance.
This is the Faraday law.
equation weight of AI = 27/3 =9
Number of equation of AI = \(\frac{\text { wt. of } \mathrm{Al}}{\text { eq. wt. }}=\frac{4.5}{9}=0.5\)
Number of Faradays required = 0.5
A number of equations of H2 produced = 0.5 eq.
The volume occupied by 1 equation of H2 = 22.4/2 = 11.2 L
Volume occupied by 0.5 eq. of H2 = 11.2 x 0.5 = 5.6 L at STP
Question 21. In electrolysis of NaCl when the Pt electrode is taken then H2 is liberated at the cathode while with H2 cathode it forms sodium amalgam. The reason for this is
- Hg is more inert than Pt
- More voltage is required to reduce H+ at Hg than at Pt
- Na is dissolved in Hg while it does not dissolve in Pt
- The cone, of H+ ions is larger when Pt electrode is taken.
Answer: 2. More voltage is required to reduce H+ at Hg than at Pt
When sodium chloride is dissolved in water, it ionizes as \(\mathrm{NaCl} \rightleftharpoons \mathrm{Na}^{+}+\mathrm{Cl}^{-} \text {. }\)
Water also dissociates as: \(\mathrm{H}_2 \mathrm{O} \rightleftharpoons \mathrm{H}^{+}+\mathrm{OH}^{-}\)
During the passing of electric current through this solution using a platinum electrode, Nan and Hn ions move toward the cathode. However, only H+ ions are discharged more readily than Na+ ions because of their low discharge potential (in the electromotive series hydrogen is lower than sodium). These H+ ions gain electrons and change into neutral atoms.
At cathode: \(\mathrm{H}^{+}+e^{-} \longrightarrow \mathrm{H}, \mathrm{H}+\mathrm{H} \longrightarrow \mathrm{H}_2\)
Cl– and OH– ions move toward the anode. Cl– ions lose electrons and change into neutral atoms.
At anode, \(\mathrm{Cl}^{-}-e^{-} \longrightarrow \mathrm{Cl}, \mathrm{Cl}+\mathrm{Cl} \longrightarrow \mathrm{Cl}_2\)
If mercury is used as the cathode, H+ ions are not discharged at the mercury cathode because mercury has a high hydrogen overvoltage. Na+ ions are discharged at the cathode in preference to H+ ions, yielding sodium, which dissolves in mercury to form sodium amalgam.
At cathode: \(\mathrm{Na}^{+}+e^{-} \longrightarrow \mathrm{Na}\)
PSEB Class 12 Chemistry Electrochemistry Questions and Answers
Question 22. A 5-ampere current is passed through a solution of zinc sulfate for 40 minutes. The amount of zinc deposited at the cathode is
- 0.4065 g
- 65.04 g
- 40.65 g
- 4.065 g
Answer: 4. 4.065 g
Current (I) = 5 ampere and time (t) = 40 minutes = 2400 seconds.
Amount of electricity passed (Q) = I x t = 5 x 2400 = 12000C
Now, \(\mathrm{Zn}^{2+}+2 e^{-} \longrightarrow \mathrm{Zn}(1 \text { mole }=65.39 \mathrm{~g})\)
Since, two charges (i.e., 2 x 96500 C) deposit 65.39 g of zinc, therefore 12000 C will deposit.
= \(\frac{65.39 \times 12000}{2 \times 96500}=4.065 \mathrm{~g} \text { of zinc }\)
Question 23. Sodium is made by the electrolysis of a molten mixture of about 40% NaCl and 60% CaCl2 because
- Ca++ can reduce NaCl to Na
- Ca++ can displace Na from NaCl
- CaCl2 helps in the conduction of electricity
- This mixture has a lower melting point than NaCl.
Answer: 4. This mixture has a lower melting point than NaCl.
Sodium is obtained by electrolytic reduction of its chloride. The melting point of chloride in sodium is high so in order to lower its melting point, calcium chloride is added to it.
Question 24. When CuSO4 is electrolyzed using platinum electrodes,
- Copper is liberated at the cathode, sulfur at the anode
- Copper is liberated at the cathode, oxygen at the anode
- Sulfur is liberated at the cathode, oxygen at the anode
- Oxygen is liberated at the cathode, and copper at the anode.
Answer: 2. Copper is liberated at the cathode, oxygen at anode
⇒ \(\mathrm{CuSO}_4 \rightleftharpoons \mathrm{Cu}^{2+}+\mathrm{SO}_4^{2-}\)
⇒ \(\mathrm{H}_2 \mathrm{O} \rightleftharpoons \mathrm{H}^{+}+\mathrm{OH}^{-}\)
At cathode: \(\mathrm{Cu}^{2+}+2 e^{-} \rightarrow \mathrm{Cu}\)
At anode: \(4 \mathrm{OH}^{-} \rightarrow 2 \mathrm{H}_2 \mathrm{O}+\mathrm{O}_2+4 e^{-}\)
PSEB Class 12 Chemistry Electrochemistry Questions and Answers
Question 25. On electrolysis of dilute sulphuric acid using platinum electrodes, the product obtained at the anode will be
- Hydrogen
- Oxygen
- Hydrogen sulphide
- Sulfur dioxide.
Answer: 2. Oxygen
During the electrolysis of dilute sulphuric acid, the product obtained at the anode will be oxygen.
At anode: \(4 \mathrm{OH}^{-} \rightleftharpoons 2 \mathrm{H}_2 \mathrm{O}+2 \mathrm{O}_2+4 e^{-}\)
Question 26. A device that converts energy of the combustion of fuels like hydrogen and methane, directly into electrical energy is known as
- Dynamo
- Ni-Cd cell
- Fuel cell
- Electrolytic cell.
Answer: 3. Fuel cell
Question 27. The efficiency of a fuel cell is given by
- ΔG/ΔS
- ΔG/ΔH
- ΔS/ΔG
- ΔH/ΔG
Answer: 2. ΔG/ΔH
The thermal efficiency, η of a fuel conversion device is the amount of useful energy produced relative to the change in enthalpy, ΔH between the product and feed streams.
= \(\frac{\text { useful energy }}{\Delta H}\)
In an ideal case of an electrochemical converter, such as a fuel cell, the change in Gibbs free energy, ΔG of the reaction is available as useful electric energy at that temperature of the conversion.
Hence, \(\eta_{\text {ideal }}=\frac{\Delta G}{\Delta H}\)
Question 28. Zinc can be coated on iron to produce galvanized iron but the reverse is not possible. It is because
- Zinc is lighter than iron
- Zinc has a lower melting point than iron
- Zinc has a lower negative electrode potential than iron
- Zinc has a higher negative electrode potential than iron.
Answer: 4. Zinc has a higher negative electrode potential than iron.
Reduction potential values of \(E_{\mathrm{Zn}^{2+} / \mathrm{Zn}}^{\circ}=-0.76 \mathrm{~V}\) and \(E_{\mathrm{Fe}^{2+} / \mathrm{Fe}}^{\circ}=-0.44 \mathrm{~V}\)
Thus, due to the higher negative electrode potential value of zinc than iron, iron cannot be coated on zinc.
Question 29. The most convenient method to protect the bottom of the ship made of iron is
- Coating it with red lead oxide
- White tin plating
- Connecting it with Mg block
- Connecting it with Pb block.
Answer: 2. White tin plating
The most convenient method to protect the bottom of the ship made of iron is white tin plating preventing the build-up of barnacles.
Question 30. To protect iron against corrosion, the most durable metal plating on it is
- Copper plating
- Zinc plating
- Nickel plating
- Tinplating.
Answer: 2. Zinc plating
