Heat Capacity, Specific Heat Capacity And Molar Heat Capacity
Heat capacity of a substance
The heat capacity is the quantity of heat required to raise the temperature ofa substance by 1°C (1 K).
The heat capacity is usually denoted by If of the amount of heat required to raise the temperature of a given amount of substance, then its heat capacity, \(C=\frac{δ q}{d T} \).
characteristics of the heat capacity of a substance
The higher the heat capacity of a substance, the smaller the increase in temperature of a given amount of the substance when a certain amount of heat is added to it. For example, the heat capacity of water is higher than that of copper. So more heat will be required to raise the temperature of 1 g of water than lg of copper by 1 K (or 1°C).
The heat capacity of a substance depends on its nature.
The heat capacity ofa substance depends on its amount. Thus, it is an extensive property.
The heat capacity is a path-dependent quantity. The heat required to raise the temperature of a substance by IK depends on the process by which the substance is heated. For example, the amount of heat required to raise the temperature of1 mol of N2 gas by IK depends on whether the heating is done at constant volume or constant pressure.
Unit: \(\text { cal } \cdot{ }^{\circ} \mathrm{C}^{-1}\left[\text { or, cal } \cdot \mathrm{K}^{-1}\right] \text { or, } \mathrm{J} \cdot{ }^{\circ} \mathrm{C}^{-1}\left[\text { or, } \mathrm{J} \cdot \mathrm{K}^{-1}\right] \text {. }\)
Read And Learn More Class 11 Chemistry Solutions
Specific heat or specific heat capacity of a substance
The amount of heat required to raise the temperature of the unit mass ofa substance by 1°C [or IK] is called specific heat or specific heat capacity ofthe substance. It is represented by d. Specific heat capacity is an intensive property ofthe system.
⇒ \(Unit: cal \cdot \mathrm{g}^{-1} \cdot{ }^{\circ} \mathrm{C}^{-1} or, cal \cdot \mathrm{g}^{-1} \cdot \mathrm{K}^{-1} or, \mathrm{J} \cdot \mathrm{g}^{-1} \cdot{ }^{\circ} \mathrm{C}^{-1} or, \mathrm{J} \cdot \mathrm{g}^{-1} \cdot \mathrm{K}^{-1}.\).
The specific heat capacity of water (4.]8J-g-1.°C-1) is considerably higher than that of other common substances. Thus, a large amount of heat as well as time is required to warm a given amount of water. For the same reason, hot water takes a long time to cool.
PSEB Class 11 Chemistry Chapter 6 Chemical Thermodynamics Notes
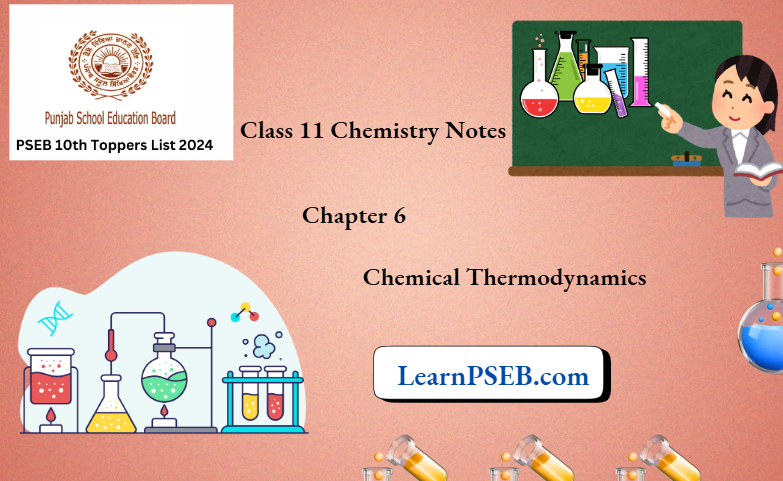
Molar heat capacity
The amount of heat required to raise the temperature of I mol of a substance by 1°C (or IK) is called the molar heat capacity of the substance. The molar heat capacity is denoted by’ Cm’ (the suffix’ m ’ refers to molar), it is an Intensive property.
⇒ \(\mathrm{cal} \cdot \mathrm{mol}^{-1} \cdot{ }^{\circ} \mathrm{C}^{-1} \text { or, cal } \cdot \mathrm{mol}^{-1} \cdot \mathrm{K}^{-1}\)
⇒ \(\text { or, } \mathrm{J} \cdot \mathrm{mol}^{-1} \cdot{ }^{\circ} \mathrm{C}^{-1} \text { or, } \mathrm{J} \cdot \mathrm{mol}^{-1} \cdot \mathrm{K}^{-1} \text {. }\)
Molar heated panacea substance(Cm)
=Specify Lear capacity of me substance(c) x Molar mass (M)
Heat absorbed or released by substance (q)
PSEB Class 11 Chemistry Chapter 6 Chemical Thermodynamics Notes
| Class 10 Science | Class 11 Chemistry |
| Class 11 Chemistry | Transformation of Sentences |
| Class 8 Maths | Class 8 Science |
= mass of the substance (m)>specificheat capacity (c) x increase or decrease in temperature <Ar> thus, q= mxcxT
[where AT =final temperature- initial temperature] Using fee equation [1], we can calculate c if we know the values of, m and AT. The heat released or absorbed (q) can be calculated by using equation [1] if we know the values of c, m, and AT. An increase or decrease in temperature (AT) can be calculated by using equation[1] if we know the values of q,c, and m.
Heat Capacity Of A Substance At Constant Volume And Pressure
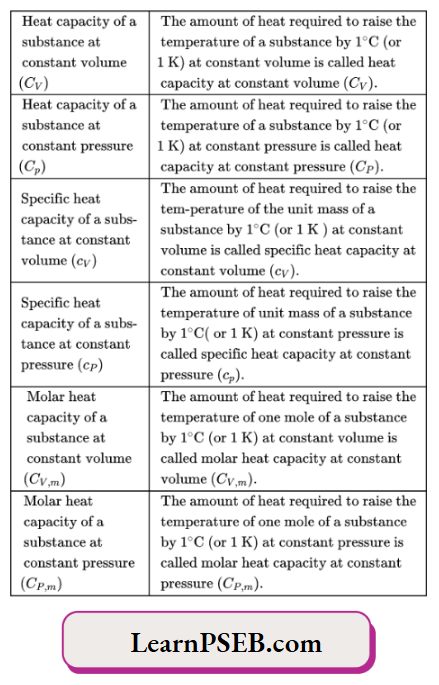
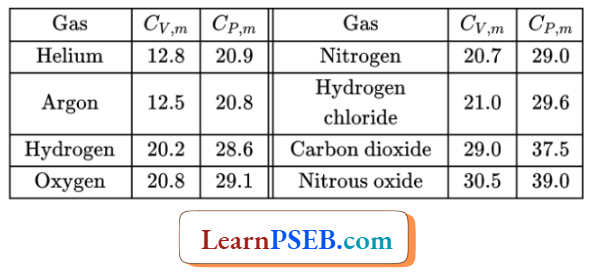
Comparison of the values of molar heat capacities at constant pressure and constant volume
Comparison for gaseous substances: For gaseous substances, the molar heat capacity at constant pressure is greater than die molar heat capacity at constant volume.
Addition of heat at constant volume: As the volume of the system is constant, no external work by the system is possible. So, all die heat added to the system will be used for increasing the internal energy of the system, which in turn increases the temperature of the die system.
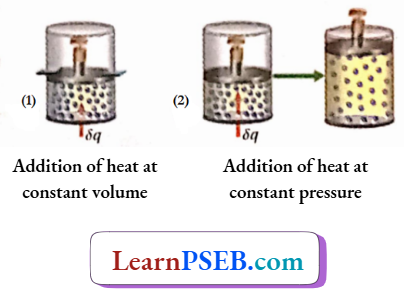
Suppose, the addition of 8a amount of heat to 1 mole of gas causes an increase in the temperature of the gas by dT. Therefore, by definition,
⇒ \(C_{V, m}=\left(\frac{\delta q}{d T}\right)_V\)
Addition of heat at constant pressure: When heat is added to a gas at constant pressure the added heat is used up in two ways. One part of it is expended for the external work done by the system, and the other part of it goes to increase the internal energy of the system.
As a result of an increase in internal energy, the temperature of the system also increases Suppose, when 5q amount of heat is added to l mole of a gas at constant pressure, the temperature of the gas is increased by dT’.
Class 11 Chemistry Chemical Thermodynamics Notes
So, by definition \(C_{P, m}=\left(\frac{\delta q}{d T^{\prime}}\right)_P\) Therefore, if Ihe same amount of heat Is added to I mol of a given gas separately at constant volume and constant pressure, then the Increase in temperature will be small at constant pressure than at constant volume so dt’,dt and cp, m. Cv, m. As a result of this, the molar heat capacity of a gas at constant pressure(cp,m) will be greater than its molar heat capacity at constant volume (cv,m).
Comparison in the case of solids and liquids: when heat is added to a solid or liquid, there occurs no significant change in their volumes. Thus, the work involved in the process of heating a solid or liquid at constant pressure is negligible. This is why the values of Cp m and Cv m are found to be almost the same in the case of a liquid or solid.
For a gas, the ratio of the molar heat capacity at constant pressure (Cp m) to the molar heat capacity at constant volume (Cp,m) is termed as heat capacity ratio (7). Therefore, 7 =CPf m/C V, m
Some relations of Cp and Cv for an ideal gas 1) Internal energy ( U) of an ideal gas depends only on the temperature (T), neither on the pressure (P) nor the volume (V). When an ideal gas undergoes a process involving only P-V work, the heat absorbed by the gas is equal to its internal energy change. Therefore, for an ideal gas
⇒ \(C_V=\left(\frac{\delta q}{d T}\right)_V=\frac{d U}{d T} \text { or, } d \boldsymbol{d}=\boldsymbol{C}_V \boldsymbol{d} T\)
Enthalpy (H) of an ideal gas depends only on temperature (T). It does not depend either on pressure (P) or on volume (V). When an ideal gas undergoes a process involving only P-V work, the heat absorbed by the gas is equal to its enthalpy change. Therefore, for an ideal gas, \(C_P=\left(\frac{\delta q}{d T}\right)_P=\frac{d H}{d T} \text { or, } d H=C_P d T\)
For an ideal gas, the difference between the moon’s heat capacities at constant pressure and constant volume is equal to the universal gas constant (R). Thus, CP, m -CV, m = R.
For 1 mole of an ideal gas, PV = RT
Substitution RT for PV in the relation H = U+ PV gives the enthalpy for1 mol of an ideal gas, i.e., H = U+ RT Differentiating both sides, we have dH = dU + RdT.
Dividing both sides by dT gives
⇒ \(\frac{d H}{d T}=\frac{d U}{d T}+R \quad \text { or, } C_{P, m}=C_{V, m}+R\) Or, \(C_{P, m}-C_{V, m}=R\)
[For an ideal gas, dU = CydT and dH = CpdT}
The change in internal energy (A) with the change in temperature of an ideal gas: Suppose, the ‘n’ mole of an ideal gas undergoes a process in which its temperature changes from T1 to T2 and so does its internal energy from U1 to U2. Therefore, the change in internal energy ofthe gas,
⇒ \(\int_{U_1}^{U_2} d U=\int_{T_1}^{T_2} n C_{V, m} d T\)
⇒ \(\text { or, } U_2-U_1=\int_{T_1}^{T_2} n C_{V, m} d T \text { or, } \Delta U=\int_{T_1}^{T_2} n C_{V, m} d T\)
Class 11 Chemistry Chemical Thermodynamics Notes
If CV,m is considered to be independent of temperature within the temperature range to T2, then | AU = nCV/Therefore, the change in internal energy (AI7) with the temperature change can be calculated by using equation [1], If T2> T1, then AU = positive, ie., with increasing temperature, the internal energy of the system increases. If T2<Ty then A U = negative i.e., with decreasing temperature, the internal energy of the system decreases.
The change in enthalpy (AH) with the change in temperature for an ideal gas: Suppose, an ideal gas undergoes a process in which its temperature changes from T1 to T2.
As a result of which its enthalpy changes from H1 to H2. Therefore, the change in enthalpy of the gas in this process is:
⇒ \(\int_{H_1}^{H_2} d H=\int_{T_1}^{T_2} n C_{P, m} d T\)
⇒ \(\text { or, } H_2-H_1=\int_{T_1}^{T_2} n C_{P, m} d T \text { or, } \Delta H=\int_{T_1}^{T_2} n C_{P, m} d T\)
If Cp m is considered to be independent of temperature within the temperature range T1 to T2, then, \(\Delta H=n C_{P, m}\left(T_2-T_1\right)\) So, the change in enthalpy (AH) with the change in temperature can be calculated by using equation [1],
Numerical Examples
Question 1. How much 90g of water from 30°C to 100°C? [Molar heat capacity of water at constant pressure = 75.3 J.mol-1 K-1]
Answer: The specific heat of water at constant pressure,
⇒ \(c_P=\frac{C_{P, m}}{M}=\frac{75.3}{18}=4.18 \mathrm{~J} \cdot \mathrm{g}^{-1} \cdot \mathrm{K}^{-1}\)
where, Cp m = molar heat capacity at constant pressure, M = molar mass ofthe substance Mass of water, m = 90 g and AT = (373-303)K =70K
∴ q =mx cpx AT = 90 x 4.18 x 70 =26334 J =26.33 kj
∴ 26.33 kl of heat is required to raise the temperature of 90g of water from 30 °C to 100 °C.
Question 2. How much heat will be released when the temperature of1 mol of water changes from 90 °C to 80 °C? Given: Specific heat of water 4.18 J.g-1.K-1
Answer: We know, q = mx cx AT The amount of water = lmol. Therefore, m = 18g, c = 4.18 J.g-1.K-1 and \(\Delta T=[(273+80)-(273+90)] \mathrm{K}=-10 \mathrm{~K}\)
∴ q = 18 X 4.18 X (-10) J = —752.40J
So, the amount of heat that will be liberated when the temperature of1 mol water changes from 90 °C to 80 °C is 752.40 J.
Question 3. Specific heats of an ideal gas at constant volume & constant pressure are 0.015 and 0.025 cal. g-1 .K-1 respectively. Determine the molar mass of the gas.
Answer: \(c_V=0.015 \mathrm{cal} \cdot \mathrm{g}^{-1} \cdot \mathrm{K}^{-1}, c_P=0.025 \mathrm{cal} \cdot \mathrm{g}^{-1} \cdot \mathrm{K}^{-1}\)
∴ Molar heat capacity at constant volume, Cy m=M x Cy, and that at constant pressure, Cp m = M x cp[Af= molar mass] Again we know, for an ideal gas, Cp m- Cy m = R.
∴ M(Cp-Cy) = R
⇒ \(\text { or, } \quad M(0.025-0.015) \mathrm{cal}^{-1} \cdot \mathrm{g}^{-1} \cdot \mathrm{K}^{-1}=1.987 \mathrm{cal}^{-1} \cdot \mathrm{K}^{-1} \cdot \mathrm{mol}^{-1}\)
or, Af = 198.7g-mol-1 \(\left[ R=1.987 \mathrm{cal} \cdot \mathrm{K}^{-1} \cdot \mathrm{mol}^{-1}\right]\)
∴ The molar mass of the gas = 198.7 g-mol-1.
Class 11 Chemistry Chemical Thermodynamics Notes
Question 3. Specific heats of an ideal gas at constant volume And constant pressure are 0.015 and 0.025 cal. g-1.K-1 respectively. Determine the molar mass of the gas.
Answer: \(c_V=0.015 \mathrm{cal} \cdot \mathrm{g}^{-1} \cdot \mathrm{K}^{-1}, c_P=0.025 \mathrm{cal} \cdot \mathrm{g}^{-1} \cdot \mathrm{K}^{-1}\)
∴ Molar heat capacity at constant volume, Cy m=M x Cy and that at constant pressure, Cp m = M x cp[Af= molar mass]
Again we know, for ideal gas, \(C_{P, m}-C_{V, m}=R.\)
∴ M(Cp-Cy) = R
Or, \(M=198.7 \mathrm{~g} \cdot \mathrm{mol}^{-1}\)
Since \(R=1.987 \mathrm{cal} \cdot \mathrm{K}^{-1} \cdot \mathrm{mol}^{-1}\)
∴ Molar mass ofthe gas = 198.7 g-mol-1.
